Acids, Bases, Salts and Oxides in Chemistry
Acids
According to Arrhenius (1887), an acid is any substance that, in aqueous solution, releases solely H+ ions. An example is hydrochloric acid (HCl):
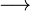 H+ + Cl–
H+ + Cl–Some years later, in 1923, Brønsted and Lowry proposed the idea that an acid is a substance that can transfer protons (H+).
This latter definition generalized Arrhenius’ theory of acids. The Brønsted-Lowry theory also serves for non-aqueous solutions. The two theories are very similar in the definition of acid, but the Brønsted-Lowry theory is much more general.
In 1923, Lewis further widened the definition of acids, a theory that did not gain acceptance until some years later. According to Lewis’ theory, an acid is any chemical species that, in any medium, can accept a pair of electrons. This definition includes substances that behave as acids but do not meet the Brønsted-Lowry definition. These are called Lewis acids. Since the proton, according to this definition, is a Lewis acid (it has an empty 1s orbital, which can accommodate a pair of electrons), one can say that all Brønsted-Lowry acids are Lewis acids, and all Arrhenius acids are Brønsted-Lowry acids.
- Examples of Brønsted-Lowry acids: HCl, HNO3, H3PO4 – these donate H+ during reactions.
- If they are in aqueous solution, they are also Arrhenius acids.
Bases
According to Svante Arrhenius, a base (also called alkali) is any substance that releases only the anion OH– (hydroxyl ions) in solution. Solutions with these properties are said to be basic or alkaline. Bases have low concentrations of H+ ions, and solutions with a pH above 7 are considered basic. They have an astringent taste and are employed as cleaners, medications (antacids), among other uses. Many bases, such as magnesium hydroxide (milk of magnesia), are weak and do not cause damage. Others, such as sodium hydroxide (NaOH or caustic soda), are corrosive, and their handling must be done carefully. When in contact with red litmus paper, they turn the paper blue or violet.
In 1923, the Danish chemist Johannes Nicolaus Brønsted and the Englishman Thomas Martin Lowry proposed the following definition: A base is an acceptor of protons (hydronium ions, H+).
Later, Gilbert Lewis defined a base as any substance that donates lone pairs of electrons in a chemical reaction (a lone pair donor).
Bases neutralize acids, according to the Arrhenius concept, forming water and a salt:
- H2SO4 + Ca(OH)2 → 2H2O + CaSO4 (sulfuric acid + calcium hydroxide → water + calcium sulfate)
- HCl + NaOH → H2O + NaCl (hydrochloric acid + sodium hydroxide → water + sodium chloride)
Salts
For other meanings of salt, see Salt (disambiguation).
In chemistry, a salt is an ionic compound, i.e., formed by cations and anions. They are typically the product of a chemical reaction between:
- A base and an acid: forming a salt and water. For example:
- 2NaOH + H2SO4 → Na2SO4 + 2H2O
- A metal and an acid: forming a salt and hydrogen. For example:
- Mg + H2SO4 → MgSO4 + H2
- A non-metal oxide and a basic oxide: forming a salt. For example:
- CO2 + CaO → CaCO3
The ions forming the salts may be monatomic (as the fluoride anion, F–, or the calcium cation, Ca2+) or polyatomic (as the sulfate anion, SO42-). They may also be inorganic (such as the aforementioned sulfate) or organic (such as the acetate anion, CH3COO–).
In general, salts form crystals. They are often soluble in water, where the ions separate. Salts generally have a high melting point, low hardness, and low compressibility. If melted or dissolved in water, they conduct electricity, as they dissociate into their constituent ions, which move and function as electrolytes.
The most commonly known salt is sodium chloride, commonly known as “common salt” or “table salt”, which is widely used in food consumption.
The neutralization of acids by bases can be total or partial, resulting in acid or basic salts.
Oxides
An oxide is a binary chemical compound composed of oxygen atoms with other elements. Oxides are a large group in chemistry since most elements form chemical oxides. Some examples of oxides that we are familiar with are rust (iron(III) oxide), carbon dioxide (carbon(IV) oxide), and lime (calcium oxide).
In oxides, the oxygen must be the most electronegative element. Compounds like OF2 or O2F2 are not oxides because fluorine is more electronegative than oxygen. These compounds are called oxygen fluorides.
