Carbohydrates: Structure, Types, and Functions
Carbohydrates: Biomolecules are formed by C, H, and O in a ratio that indicates its empirical formula CnH2nOn, although exceptionally they may contain atoms of H, S, and P. These compounds may represent up to 90% of the biomolecules organic in the body, hence its importance. Also known as carbohydrates because they were initially thought to be formed by the structure of carbonated and hydrated water molecules. Chemically, the carbohydrates are aldoses or ketones with multiple hydroxyl groups, although more complex may contain other functional groups-OH (alcohol),-NH2 (amine).
The simplest carbohydrates are called monosaccharides. These are formed by the union of a variable amount of monosaccharides and may even join with lipids or proteins. Monosaccharides can be classified as: Holosides: Monosaccharides are formed only by bears. According to the number of monomers together, there are different oligosaccharides, which contain between 2 and 10 monosaccharides, and disaccharides are the most notable. Polysaccharides are composed of multiple repeating units of monosaccharides, and in turn, are divided into homopolysaccharides formed by the repetition of 1 monomer and heteropolysaccharides, which are more varied since they have more than 1 monomer. Heterosides: Combination of all monkeys and other molecules not carbohydrate, may be glycolipids and glycoproteins.
Monosaccharides are white crystalline solids, soluble in water, and have a reductive character. They have a characteristic sweet taste, which is why they are known as sugars. They contain between 3 and 7 carbon atoms, named after triose (glyceraldehyde), tetra (erythrose), pentoses (ribose), hexoses (glucose), or heptoses. Polyols are chemically bonded to an aldehyde or ketone group; according to the functional group, they can be aldoses (aldehyde C1) or ketoses (ketone C2).
Disaccharides: Results from the union of 1 covalent bond + 2 monosaccharides; this is called a glycosidic bond. In the molecule, water is released, and it can be mono- or dicarbonyl, and the position of the -OH of the anomeric carbon of the first monosaccharide can be a function. The most common are: Maltose (malt sugar) formed by 2 molecules of glucose. Lactose (sugar of lexemes) is the union of 1 glucose + 1 galactose. Sucrose (cane sugar) is formed by 1 molecule of glucose + 1 molecule of fructose.
In the field of carbohydrates, the link is the O-glycosidic bond to unite monosaccharides in order to form disaccharides or polysaccharides.
Through this link, two monosaccharides are joined by the following scheme:
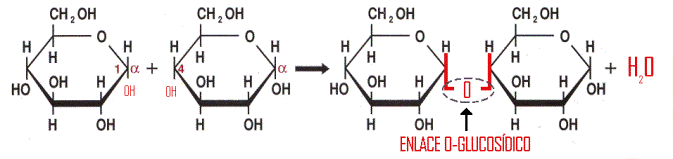
Polysaccharides: Polysaccharides are biomolecules formed by the union of a large number of monosaccharides. They fall between carbohydrates and fulfill various functions, especially energy reserves and structural. Polysaccharides are polymers whose constituent monomers are monosaccharides, which repeatedly join by glycosidic bonds. The polysaccharides can be broken down by hydrolysis of glycosidic bonds between residues in polysaccharides. Digestion within the cells or digestive cavities is a digestive enzyme-catalyzed hydrolysis called glycosidases, which are specific for certain polysaccharides and, especially, for certain types of glycosidic bonds. In the formation of each glycosidic bond, a water molecule is released, and in its break by hydrolysis, one molecule of water is consumed, so in a chain made of n monosaccharides, there will be n-1 glycosidic bonds.
