Coordination Compounds: Roles in Biology, Chemistry, and Metallurgy
Coordination Compounds: Applications and Stability
9.27. Discuss briefly, giving an example in each case, the role of coordination compounds in:
(a) Biological systems
(b) Analytical chemistry
(c) Medicinal chemistry
(d) Extraction/metallurgy of metals
Answer:
(i) Coordination compounds are of great importance in biological systems. The pigment responsible for photosynthesis, chlorophyll, is a coordination compound of magnesium. Haemoglobin, the red pigment of blood which acts as an oxygen carrier, is a coordination compound of iron. Vitamin B12, cyanocobalamin, the anti-pernicious anaemia factor, is a coordination compound of cobalt. Among the other compounds of biological importance with coordinated metal ions are enzymes like carboxypeptidase A and carbonic anhydrase (catalysts of biological systems).
(ii) There is growing interest in the use of chelate therapy in medicinal chemistry. An example is the treatment of problems caused by the presence of metals in toxic proportions in plant/animal systems. Thus, excess copper and iron are removed by the chelating ligands D-penicillamine and desferrioxime B via the formation of coordination compounds.
EDTA is used in the treatment of lead poisoning. Some coordination compounds of platinum effectively inhibit the growth of tumours. Examples are cis-platin and related compounds.
(iii) Coordination compounds find use in many qualitative and quantitative chemical analyses. The familiar colour reactions given by metal ions with a number of ligands (especially chelating ligands), as a result of the formation of coordination entities, form the basis for their detection and estimation by classical and instrumental methods of analysis. Examples of such reagents include EDTA, DMG (dimethylglyoxime), α-nitroso-β-naphthol, cupron, etc.
(iv) Some important extraction processes of metals, like those of silver and gold, make use of complex formation. Gold, for example, combines with cyanide in the presence of oxygen and water to form the coordination entity [Au(CN)2]– in aqueous solution. Gold can be separated in metallic form from this solution by the addition of zinc.
9.26. What is meant by the chelate effect? Give an example.
Answer: When a didentate or a polydentate ligand contains donor atoms positioned in such a way that when they coordinate with the central metal ion, a five- or six-membered ring is formed, the effect is called the chelate effect. For example:
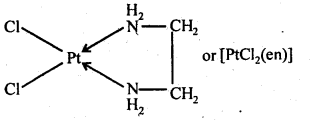
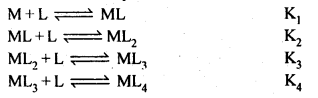
What is meant by the stability of a coordination compound in solution? State the factors which govern the stability of complexes. Formation of a complex in solution is an equilibrium reaction. It may be represented as:
M+4L ⇌ ML4
The equilibrium constant of this reaction is the measure of the stability of the complex. Hence, the equilibrium constant is also called the stability constant. The instability constant may be defined as the equilibrium constant for the reverse reaction. The formation of the above complex may also be written in successive steps:
The stability constant is written as β4 = K1K2K3K4.
Greater the stability constant, stronger is the metal-ligand bond.
The stability of a complex will depend on:
(a) Nature of metal (b) Oxidation state of metal (c) Nature of ligand, e.g., chelating ligands form stabler complexes (d) Greater the basic strength of the ligand, the more will be the stability.
