Fundamental Chemical Laws and Concepts
The Law of Conservation of Mass
The law of conservation of mass, also known as the law of conservation of matter or the Lomonosov-Lavoisier law, states that in an ordinary chemical reaction, the mass remains constant. That is, the mass of the reactants consumed is equal to the mass of the products obtained.
Dalton’s Law (Law of Multiple Proportions)
Dalton’s law, or the law of multiple proportions, formulated in 1803 by John Dalton, is one of the most fundamental stoichiometric laws. It was demonstrated by French chemist and physicist Joseph Louis Gay-Lussac.
Law of Equivalent Proportions
The law of equivalent proportions, also called the law of combining weights, law of reciprocal proportions, or Richter-Wenzel law, states that the weights of different elements that combine with the same weight of a given element are the relative weights of those elements when they combine with each other, or multiples or fractions of these weights.
Molar Volume
The molar volume of a substance, symbolized Vm,[1] is the volume occupied by one mole of that substance. The unit in the International System of Units is the cubic meter per mole: m3 · mol-1.
Moles and Avogadro’s Number
One mole of any substance contains 6.022 × 1023 particles.[2] In the case of molecular gases, one mole contains NA molecules. From this, considering Avogadro’s law, it follows that one mole of any gaseous substance will always occupy the same volume (measured under the same conditions of pressure and temperature).
Molar Volume of Ideal Gases
Experimentally, it has been shown that the volume occupied by one mole of any ideal gas at standard conditions (pressure = 1 atm, Temperature = 273.15 K = 0 °C) is 22.4 liters.[3] This value is known as the normal molar volume of a gas.
This value corresponds to the molar volume of ideal or perfect gases. Ordinary gases are not perfect (their molecules have a certain volume, however small), and their molar volume deviates slightly from this value. For example, the molar volumes of some gases are:
- Carbon monoxide (CO) = 22.4 L
- Sulfur dioxide (SO2) = 21.9 L
- Carbon dioxide (CO2) = 22.3 L
For substances in solid or liquid states, the molar volume is much smaller and different for each substance. For example:
- For liquid nitrogen (-210 °C), the molar volume is 34.6 cm3.
- For liquid water (4 °C), the molar volume is 18.0 cm3.
Atomic Mass
The atomic mass (ma) is the mass of an atom, most often expressed in unified atomic mass units.[1] The atomic mass can be considered the total mass of protons and neutrons in an atom (when the atom is not moving). The atomic mass is sometimes incorrectly used as a synonym for relative atomic mass, average atomic mass, and atomic weight; however, the latter differ subtly from the atomic mass. The atomic mass is defined as the mass of a single atom, which can only be of one isotope at a time, and is not a weighted average of the abundances of the isotopes.
The Mole
The mole (symbol: mol) is the unit that measures the amount of a substance. It is one of the seven fundamental physical quantities of the International System of Units.
1 mol = 6.02214179(30) × 1023
Relative Molecular Mass
The relative molecular mass is a number that indicates how many times greater the mass of one molecule of a substance is than the unified atomic mass unit. Its unit is the Dalton or atomic mass unit, abbreviated u (formerly amu).
Calculating Molecular Mass
Molecular mass is determined by adding the relative atomic masses of the elements whose atoms form a molecule of that substance. Although it is popularly called molecular weight, the correct term is molecular mass. The molar mass of a substance coincides numerically with the molecular mass, although they represent different concepts.
The formula for calculating the percentage of an element X is: % element X = [(No. of X atoms) · Ar(X) / Mr] * 100%
The molecular mass is calculated by summing the atomic masses of the elements in the molecule. For example, for the water molecule, H2O, the molecular mass is:
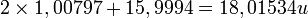
(Atomic mass of H: 1.00797, atomic mass of O: 15.9994)
The atomic mass of hydrogen is multiplied by 2 because the water molecule contains two hydrogen atoms (H).
