Molar Mass of Solute and Sulfuric Acid Manufacturing Process
An aqueous solution of 2% non-volatile solute exerts a pressure of 1.004 bar at the normal boiling point of the solvent. What is the molar mass of the solute?
Here,
Vapour pressure of the solution at normal boiling point (p1) = 1.004 bar (Given)
Vapour pressure of pure water at normal boiling point (p10) = 1.013 bar
Mass of solute, (w2) = 2 g
Mass of solvent (water), (w1) = 100 – 2 = 98 g
Molar mass of solvent (water), (M1) = 18 g mol – 1
According to Raoult’s law,
(p10 – p1) / p10 = (w2 x M1 ) / (M2 x w1 )
(1.013 – 1.004) / 1.013 = (2 x 18) / (M2 x 98 )
0.009 / 1.013 = (2 x 18) / (M2 x 98 )
M2 = (2 x 18 x 1.013) / (0.009 x 98)
M2 = 41.35 g mol – 1
Hence, the molar mass of the solute is 41.35 g mol – 1 What is the order of the reaction with respect to A and B?
AnswerLet the order of reactant A = x
And the order of reactant B = y
We have the formula
Take log both side we get
log 2.821 = log 2 x
take the log of 2.821 and Use formula log ax = x log a we get
0.4504 = x log 2
Value of log 2 = 0.3020
We get
x = 0.4504/0.3020 = 1.5(approx)
Answer
The order of reactant A = 1.5
The order of reactant B = 0
manufacture of sulfuric acid: Introduction It was once said that a country’s wealth could be measured by its production of sulfuric acid (H2SO4). That may no longer be true, but the acid is still used in the manufacture of paints, fertilizers, plastics, fabrics, dyes, detergents, and many other useful products. In this unit you can find out how we make the acid in industry The Contact process The most important process for making sulfuric
acid in industry is the Contact process. We can think of the Contact process as involving three stages. Look at the process shown in Fig.1 below: Stage 1Sulfur is imported from Poland or the USA. We can also obtain sulfur from the impurities in fossil fuels such as coal. In the first stage of the process, sulfur is burned in air to make sulfur dioxide gas:sulfur + oxygen  sulfur dioxide
sulfur dioxide
 SO2(g) Stage 2 In the next stage, we convert the sulfur dioxide to sulfur trioxide: sulfur dioxide + oxygen
SO2(g) Stage 2 In the next stage, we convert the sulfur dioxide to sulfur trioxide: sulfur dioxide + oxygen  sulfur trioxide
sulfur trioxide2 SO2(g) + O2(g)  2 SO3(g)The reaction happens on a catalyst of vanadium(V) oxide to speed up the reaction. As much sulfur dioxide as possible is changed into sulfur trioxide, and releases of sulfur dioxide are prevented because it is a gas that causes acid rain.Stage 3 In the final stage, the sulfur trioxide is converted into sulfuric acid. The sulfur trioxide gas is absorbed into very concentrated sulfuric acid (a 98 per cent solution of H2SO4in water), producing a thick fuming liquid called oleum. The oleum is mixed carefully with water, and the sulfur trioxide in the oleum reacts with the water as follows: sulfur trioxide + water
2 SO3(g)The reaction happens on a catalyst of vanadium(V) oxide to speed up the reaction. As much sulfur dioxide as possible is changed into sulfur trioxide, and releases of sulfur dioxide are prevented because it is a gas that causes acid rain.Stage 3 In the final stage, the sulfur trioxide is converted into sulfuric acid. The sulfur trioxide gas is absorbed into very concentrated sulfuric acid (a 98 per cent solution of H2SO4in water), producing a thick fuming liquid called oleum. The oleum is mixed carefully with water, and the sulfur trioxide in the oleum reacts with the water as follows: sulfur trioxide + water  sulfuric acid
sulfuric acid
SO3(g) + H2O(l)  H2SO4(l) You may wonder why the sulfur trioxide is not mixed directly with pure water. The problem is that this is a highly exothermic reaction, which would produce a fine mist of sulfuric acid that is difficult to condense and could escape to pollute the air.
H2SO4(l) You may wonder why the sulfur trioxide is not mixed directly with pure water. The problem is that this is a highly exothermic reaction, which would produce a fine mist of sulfuric acid that is difficult to condense and could escape to pollute the air.

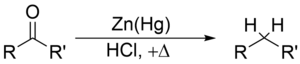 @ Sandmeyer reaction The Sandmeyer reaction is a chemical reactionused to synthesize aryl halides from aryl diazonium salts using copper salts as reagents or catalysts. It is an example of a radical-nucleophilic aromatic substitution.
@ Sandmeyer reaction The Sandmeyer reaction is a chemical reactionused to synthesize aryl halides from aryl diazonium salts using copper salts as reagents or catalysts. It is an example of a radical-nucleophilic aromatic substitution.